Research Highlights
Five Birds With One Stone: Photoelectron Photoion Coincidence Unveils Rich Phthalide Pyrolysis Chemistry
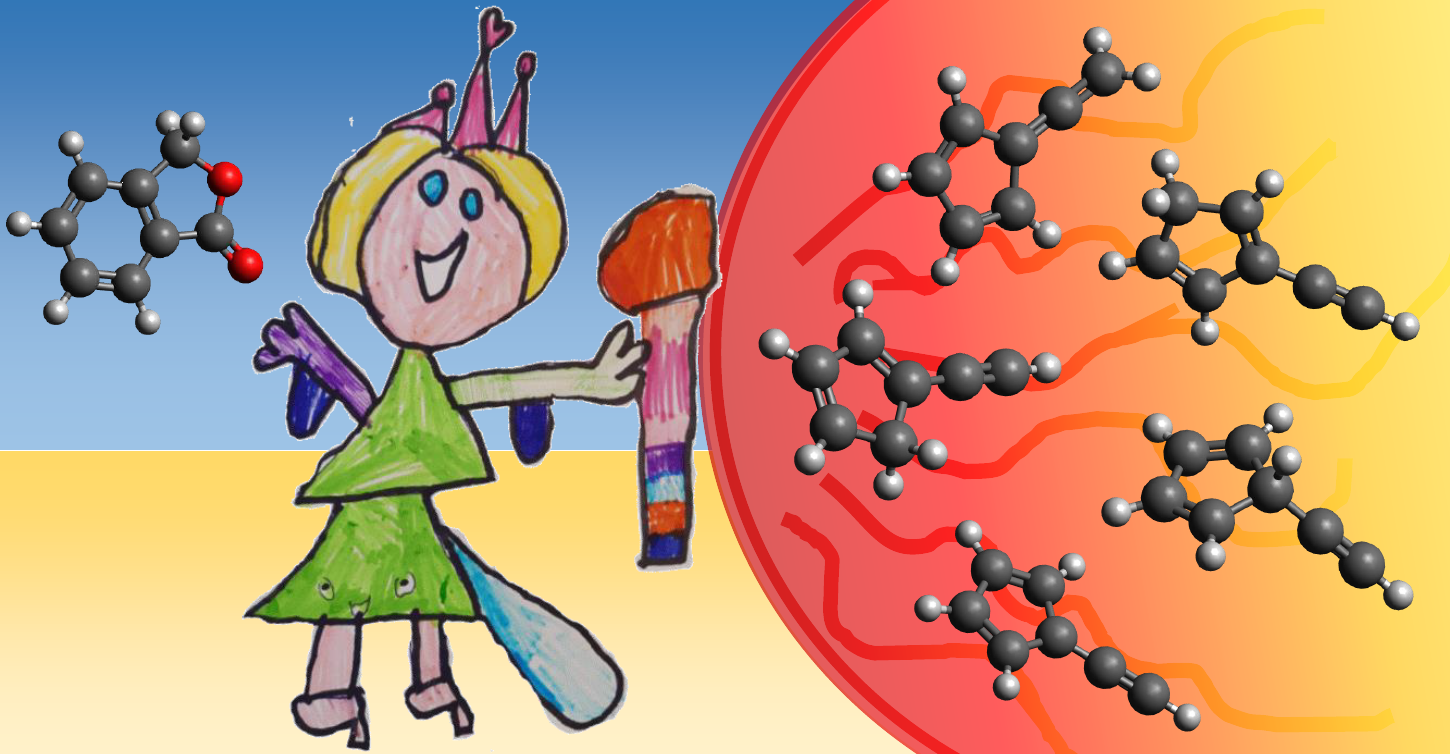 Molecules with a mass-to-charge ratio of 90 amu are commonly detected
as reaction products in combustion and flame experiments. The pentagon-bearing molecules fulvenallene, 1-ethynylcyclopentadiene,
2-ethynylcyclopentadiene, and 5-ethynylcyclopentadiene are four energetically favorable isomers of C7H6 composition that can account for this signal.
The nature of the m/z 90 species formed in combustion, however, is unknown due to the fact that the ionization energies of the potential carriers have not been determined experimentally.
Moreover, the computed ionization energies of fulvenallene and 1-ethynylcyclopentadiene are too close in energy to make a conclusive assignment.
In this work, we pyrolyze phthalide and record the photoion mass-selected threshold photoelectron spectra (TPES) of the product species at
two temperatures. Based on Franck-Condon simulations of the TPE spectra we were able to assign the ionization energies of the four isomers as
well as identify electronically excited ionic states. The C7H5 fulvenalleny radical forms at higher temperatures and its ground- and excited
states have also been characterized from the TPES. The ionization potentials and excited state features allow for these species to be identified
in flame experiments to assess their role in the growth of polycyclic aromatic hydrocarbons and soot formation.
The work is published in the Journal of Physical Chemistry A, but unfortunately without the marvelous artwork made by Zoey Sophie Bouwman.
Molecules with a mass-to-charge ratio of 90 amu are commonly detected
as reaction products in combustion and flame experiments. The pentagon-bearing molecules fulvenallene, 1-ethynylcyclopentadiene,
2-ethynylcyclopentadiene, and 5-ethynylcyclopentadiene are four energetically favorable isomers of C7H6 composition that can account for this signal.
The nature of the m/z 90 species formed in combustion, however, is unknown due to the fact that the ionization energies of the potential carriers have not been determined experimentally.
Moreover, the computed ionization energies of fulvenallene and 1-ethynylcyclopentadiene are too close in energy to make a conclusive assignment.
In this work, we pyrolyze phthalide and record the photoion mass-selected threshold photoelectron spectra (TPES) of the product species at
two temperatures. Based on Franck-Condon simulations of the TPE spectra we were able to assign the ionization energies of the four isomers as
well as identify electronically excited ionic states. The C7H5 fulvenalleny radical forms at higher temperatures and its ground- and excited
states have also been characterized from the TPES. The ionization potentials and excited state features allow for these species to be identified
in flame experiments to assess their role in the growth of polycyclic aromatic hydrocarbons and soot formation.
The work is published in the Journal of Physical Chemistry A, but unfortunately without the marvelous artwork made by Zoey Sophie Bouwman.
Structural investigation of doubly-dehydrogenated pyrene cations
 In interstellar regions that are illuminated by strong UV fields, PAHs can ionize and even dissociate. One common PAH decomposition mechanism is the loss
of molecular hydrogen. We set out to reveal the molecular structure of the dissociation product resulting from H2 loss from
a prototypical pericondensed PAH species, pyrene (C16H10). We employed infrared predissociation spectroscopy
in a cryogenic 22-pole ion trap instrument at the free electron laser for infrared experiments (FELIX) to record a well-resolved
infrared fingerprint spectrum of the mass-isolated (neon-tagged) dissociation products. Comparison with
Density Functional Theory (DFT) computed IR spectra revealed that the 1,2- and 3,4- doubly dehydrogenated Pyrene (ddPy+)
isomers are abundantly formed and that a contribution by the 1,3-ddPy isomer cannot be ruled out. The study shows that hydrogen roaming
from one ring to an adjacent aromatic ring prior to dissociation is inhibited. Furthermore, the study sheds light on the potential role that
small PAHs have on hydrogen formation in strongly illuminated interstellar objects. The work is available as a HOT article from Physical Chemistry Chemical Physics. Figure courtesy of first author Sanjana Panchagnula.
In interstellar regions that are illuminated by strong UV fields, PAHs can ionize and even dissociate. One common PAH decomposition mechanism is the loss
of molecular hydrogen. We set out to reveal the molecular structure of the dissociation product resulting from H2 loss from
a prototypical pericondensed PAH species, pyrene (C16H10). We employed infrared predissociation spectroscopy
in a cryogenic 22-pole ion trap instrument at the free electron laser for infrared experiments (FELIX) to record a well-resolved
infrared fingerprint spectrum of the mass-isolated (neon-tagged) dissociation products. Comparison with
Density Functional Theory (DFT) computed IR spectra revealed that the 1,2- and 3,4- doubly dehydrogenated Pyrene (ddPy+)
isomers are abundantly formed and that a contribution by the 1,3-ddPy isomer cannot be ruled out. The study shows that hydrogen roaming
from one ring to an adjacent aromatic ring prior to dissociation is inhibited. Furthermore, the study sheds light on the potential role that
small PAHs have on hydrogen formation in strongly illuminated interstellar objects. The work is available as a HOT article from Physical Chemistry Chemical Physics. Figure courtesy of first author Sanjana Panchagnula.
Nitrogen matters: Difference between formation of nitrogen bearing and non-nitrogen bearing polyaromatics
 Polycyclic aromatic hydrocarbons (PAHs)
play an important role in the interstellar medium and planetary atmospheres. From interstellar PAH mid-infrared emission bands it has been
estimated that PAHs lock up some 15% of the total cosmic carbon budget, emphasizing their importance in the cosmic cycle of matter. A shift
of the 6.2 micrometer emission mode is explained by the inclusion of nitrogen atoms in the otherwise homocyclic carbon-containing aromatic
molecules. While the formation of carbon-homocyclic aromatic molecules such as naphthalene has been studied in great detail, the formation
of heterocyclic species such as quinoline has received much less attention. We studied the products formed in a pyrolysis microreactor from
the phenyl+acrylonitrile reaction by means of threshold photoelectron photoion coincidence spectroscopy. Using this approach we make isomer
specific product assignments and find that the N-containing aromatic molecule quinoline does not form. This is in stark contrast to the
analogous and isoelectronic phenyl+vinylacetylene reaction which was found to yield significant fraction of naphthalene. Quantum chemical
computations show that the stability of the nitrile group prevents the second ring from closing. The work is published in Physical Chemistry
Chemical Physics.
Polycyclic aromatic hydrocarbons (PAHs)
play an important role in the interstellar medium and planetary atmospheres. From interstellar PAH mid-infrared emission bands it has been
estimated that PAHs lock up some 15% of the total cosmic carbon budget, emphasizing their importance in the cosmic cycle of matter. A shift
of the 6.2 micrometer emission mode is explained by the inclusion of nitrogen atoms in the otherwise homocyclic carbon-containing aromatic
molecules. While the formation of carbon-homocyclic aromatic molecules such as naphthalene has been studied in great detail, the formation
of heterocyclic species such as quinoline has received much less attention. We studied the products formed in a pyrolysis microreactor from
the phenyl+acrylonitrile reaction by means of threshold photoelectron photoion coincidence spectroscopy. Using this approach we make isomer
specific product assignments and find that the N-containing aromatic molecule quinoline does not form. This is in stark contrast to the
analogous and isoelectronic phenyl+vinylacetylene reaction which was found to yield significant fraction of naphthalene. Quantum chemical
computations show that the stability of the nitrile group prevents the second ring from closing. The work is published in Physical Chemistry
Chemical Physics.
Spectroscopic characterization of adamantane dissociation products
 Diamondoids are interesting species as they are constructed from diamond unit-cells terminated by hydrogen atoms.
Diamondods are also thought to be present in the interstellar medium, where they undergo strong energetic processing. We investigated the dissociation products that
form from electron ionization of adamantane by means of infrared multiphoton dissociation (IRMPD) spectroscopy in a quadrupole ion trap connected to the free electron laser (FELIX).
From a detailed comparisson with density functional theory computed IR spectra we find that a variety of carbocations are formed. The work is available from ChemPhysChem.
Diamondoids are interesting species as they are constructed from diamond unit-cells terminated by hydrogen atoms.
Diamondods are also thought to be present in the interstellar medium, where they undergo strong energetic processing. We investigated the dissociation products that
form from electron ionization of adamantane by means of infrared multiphoton dissociation (IRMPD) spectroscopy in a quadrupole ion trap connected to the free electron laser (FELIX).
From a detailed comparisson with density functional theory computed IR spectra we find that a variety of carbocations are formed. The work is available from ChemPhysChem.
Facile pentagon formation in the dissociation of aromatics
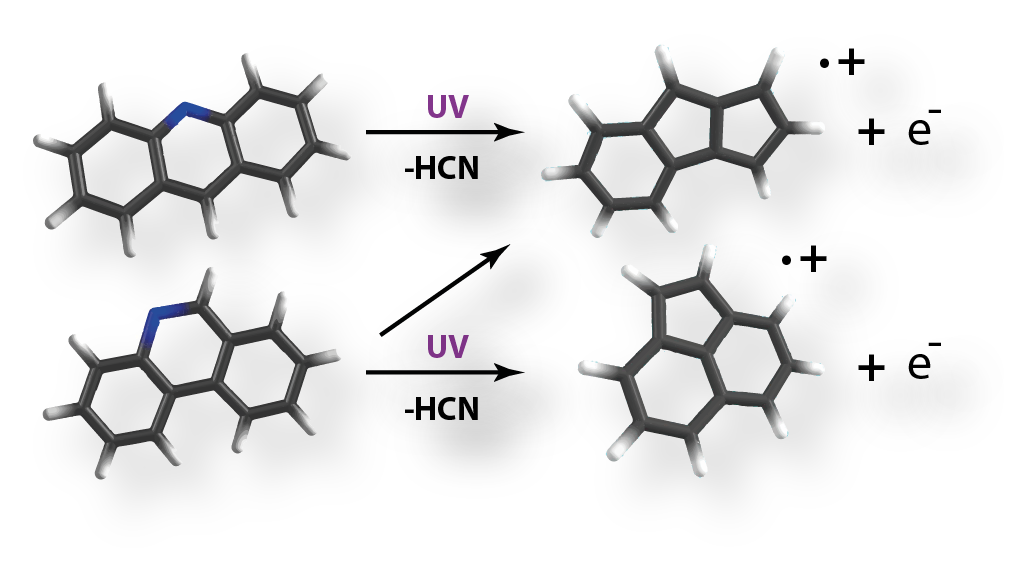 Energetic processing of
(nitrogen containing) aromatic hydrocarbons (PA(N)Hs) is believed to occur in photon dominated regions.
Observational evidence points to photoprocessing of large interstellar PAHs being responsible for the formation
of fullerene like species, such as C60. We have shown for the first time, by a combination of tandem mass spectrometry,
infrared multiphoton dissociation spectroscopy and quantum chemical computations, that pentagon formation is common
in the dissociation of two small model aromatics containing of three fused rings. Pentagon formation comprises a
crucial step in fullerene formation and thus this work provides the first experimental link between interstellar PAHs
and fullerenes. The work is available from Physical Chemistry Chemical Physics.
Energetic processing of
(nitrogen containing) aromatic hydrocarbons (PA(N)Hs) is believed to occur in photon dominated regions.
Observational evidence points to photoprocessing of large interstellar PAHs being responsible for the formation
of fullerene like species, such as C60. We have shown for the first time, by a combination of tandem mass spectrometry,
infrared multiphoton dissociation spectroscopy and quantum chemical computations, that pentagon formation is common
in the dissociation of two small model aromatics containing of three fused rings. Pentagon formation comprises a
crucial step in fullerene formation and thus this work provides the first experimental link between interstellar PAHs
and fullerenes. The work is available from Physical Chemistry Chemical Physics.
Formation of pentalene from the dissociation of naphthalene
 The loss of an acetylene unit constitutes one of the major dissociation pathways of irregular PAHs, yet the structures resulting from this unimolecular dissociation havd remained elusive for a long time. Using Infrared Multiphoton Dissociation (IRMPD) on mass selected species trapped in a quadrupole ion trap apparatus we have studied the dissociative ionization of naphthalene. From the spectra, we concluded that the pentalene cation is formed upon dissociative ionization of the smallest PAH, naphthalene. The underlying hexagon to pentagon formation may play a crucial role in the formation of interstellar buckminsterfullerenes by photodissociaion of large PAHs. The work is published in Chemical Communications.
The loss of an acetylene unit constitutes one of the major dissociation pathways of irregular PAHs, yet the structures resulting from this unimolecular dissociation havd remained elusive for a long time. Using Infrared Multiphoton Dissociation (IRMPD) on mass selected species trapped in a quadrupole ion trap apparatus we have studied the dissociative ionization of naphthalene. From the spectra, we concluded that the pentalene cation is formed upon dissociative ionization of the smallest PAH, naphthalene. The underlying hexagon to pentagon formation may play a crucial role in the formation of interstellar buckminsterfullerenes by photodissociaion of large PAHs. The work is published in Chemical Communications.
Formation of cyclopentadiene in the allyl+acetylene reaction
The formation of PAHs in combustion environments has been extensively studied, yet the chemical mechanisms
leading to the 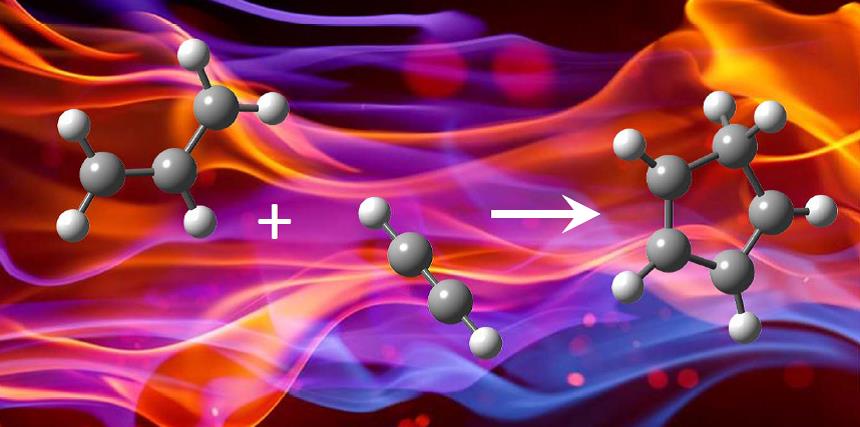 formation of the first aromatic
rings is still not completely understood. It has been proposed that resonantly stabilized radicals play a pivotal role in the
formation of aromatics. One of these radicals, the allyl (C3H5) radical, has been proposed to yield a cyclic hydrocarbon by
reaction with acetylene. Direct spectroscopic evidence for this mechanism is largely lacking from the literature. Using threshold
photoelectron spectroscopy on the iPEPICO machine at Vacuum Ultraviolet Beamline of the the Swiss Light Source (SLS) we found that cyclopentadiene is indeed formed from this
reaction. The results have been published in Physical Chemistry Chemical Physics.
formation of the first aromatic
rings is still not completely understood. It has been proposed that resonantly stabilized radicals play a pivotal role in the
formation of aromatics. One of these radicals, the allyl (C3H5) radical, has been proposed to yield a cyclic hydrocarbon by
reaction with acetylene. Direct spectroscopic evidence for this mechanism is largely lacking from the literature. Using threshold
photoelectron spectroscopy on the iPEPICO machine at Vacuum Ultraviolet Beamline of the the Swiss Light Source (SLS) we found that cyclopentadiene is indeed formed from this
reaction. The results have been published in Physical Chemistry Chemical Physics.